Executive Summary
We are at a time when there is immense pressure to accelerate drug development timelines and get drugs to market faster and increasing financial pressures on biotech. This is especially true in rare diseases and rare oncology where patient numbers are few. De-risking these investments requires a scientific and thoughtful approach to understanding disease processes better, preferably with high-quality, fit-for-purpose real-world data (RWD). While traditionally people have relied on existing retrospective data and literature reviews, these provide limited insight into disease progression, and patient preferences. The heterogeneity of these data challenge regulatory submissions and confound trial design.
This paper outlines how biotech companies can conduct natural history studies (NHS) by leveraging Pulse Infoframe’s healthie™ platform, a regulatory-grade, agile, and extensible solution built for the full lifecycle of drug development.
Sponsors using healthie™ benefit from:
- 40–50% lower labor and technology costs versus traditional models
- Faster, more efficient startup with fit-for-purpose study templates that streamline design and approval while maintaining scientific rigor. Clear contracting structure and a lean, expert support team to reduce delays and change orders
- healthie™ replaces siloed vendors and static datasets with a dynamic, real-world registry that evolves alongside your program, from preclinical to long term follow-up
1. Understanding Natural History Studies (NHS)
A Natural History Study (NHS) follows a group of individuals with a specific disease over time, collecting clinical, patient-reported, and real-world data (RWD) to understand disease progression, variability, and potential treatment pathways. Often this this done ambispectively, collecting data retrospectively and hen following patients into the future.
In rare diseases, where patient populations are small and literature is sparse, NHS plays a critical role in:
- Defining disease heterogeneity and progression
- Supporting endpoint validation and development of trial design
- Establishing external control arms
- Supporting pricing, reimbursement, and post-marketing activities
The Food and Drug Administration (FDA) and European Medicines Agency (EMA) explicitly recognize NHS as part of the regulatory toolkit for accelerated pathways, especially in rare and ultra-rare indications. (Source: FDA Guidance for Industry, Rare Diseases: Natural History Studies for Drug Development)
2. Limitations of Traditional NHS Models
Many natural history studies still rely on static real-world data, claims, electronic medical records (EMRs), or literature reviews. While adequate for initial scoping, these sources have limitations due to publication bias, costly to operationalize, inflexible, and siloed approaches. Key elements such as imaging, patient reported outcome measures (PROs), and underrepresented patient subgroups are often missing, creating blind spots that slow development and delay patient access to therapies.
Literature reviews are often used as a faster and cheaper alternative, but they rarely meet modern regulatory standards. They exclude failed trials and atypical cases, overrepresent positive studies, and exclude failed studies. This skews understanding of disease burden, natural variation, and treatment response, risking suboptimal design decisions.
Key Points
- Legacy reliance: Static RWD and literature reviews lack flexibility, completeness, and the efficiency of a fit-for-purpose approach.
- Data gaps: Missing PROs, imaging, and rare patient subgroups hinder regulatory-grade insight.
- Bias risk: Literature reviews exclude failures and outliers, skewing disease understanding.
- Platform advantage: healthie™ integrates multi-source, multi-use data capture in a single environment, ensuring high data-fidelity.
- Fit-for-purpose design: Configured to each sponsor’s objectives for greater efficiency, relevance, and reusability across the product lifecycle.
Pulse’s benchmarking shows that traditional delivery models tend to be less agile, more labor-intensive, and single use. The healthie™ platform-based registry model supports multi-use, integrated datasets that combine prospective and retrospective capture in one environment, ensuring completeness across all data from the outset.
With built-in feasibility assessment, protocol development, multilingual configuration, and real-time dashboards, healthie™ moves studies from concept to live deployment in significantly less time than the traditional approach. Sponsors gain regulatory-grade evidence that can flex to evolving research needs, informing early development, regulatory filings, HTA submissions, and post-marketing follow-up without rebuilding infrastructure.
3. When to Choose a Natural History Study
Based on Pulse’s internal decision framework, a fit-for-purpose NHS is the preferred strategy when:
- Clinical precedent is limited or evolving
- The sponsor needs regulatory-grade, prospective data
- Literature and static RWD lack specificity or are not fit-for-purpose
- PROs, biomarkers, or imaging outcomes are essential
- The study may evolve into a post-marketing, HEOR, or LTFU application
Conversely, static RWD or literature may be appropriate for early exploration but should not be relied upon for pivotal decisions without proper scientific and operational alignment.
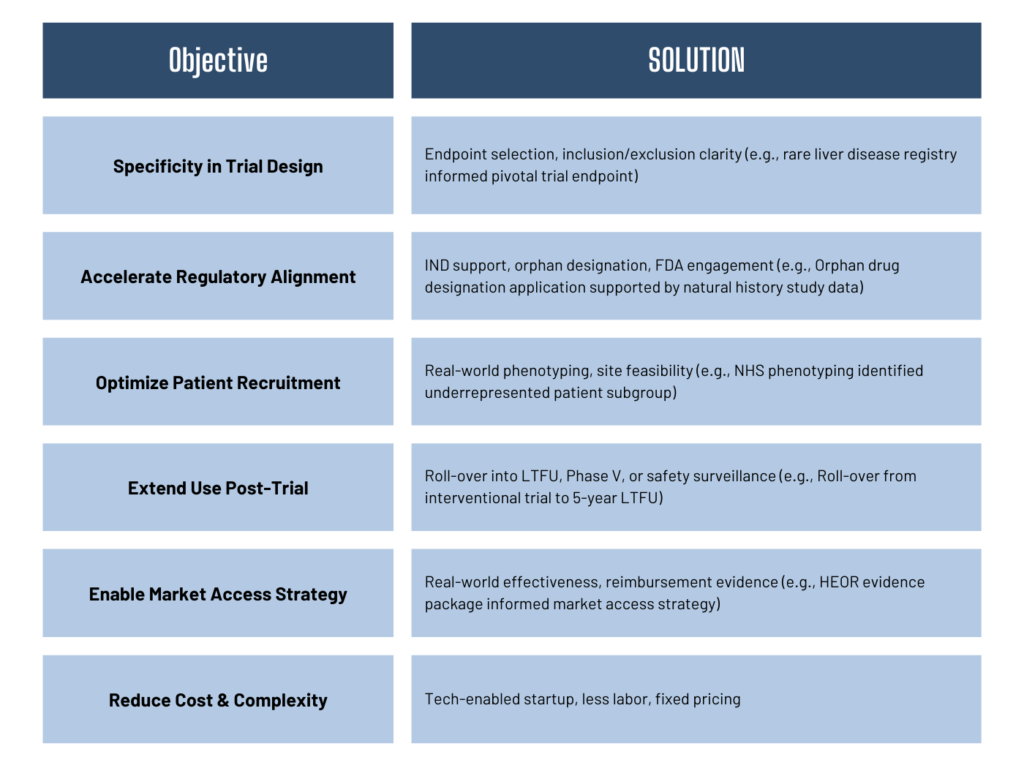
4. Strategic Benefits for Biotech Sponsors
5. Pulse Infoframe’s healthieTM Platform: A Technology-First Approach
The healthie™ platform delivers a high-quality, modular, and extensible infrastructure for conducting prospective natural history studies at significantly lower cost and labor burden than traditional, fragmented approaches. Its digital-first, fit-for-purpose design includes disease-specific eCRFs and eCOAs, integrated eConsent and ePRO, wearable/device connectivity, and robust regulatory compliance (CDISC, 21 CFR Part 11, GDPR, HIPAA). Templated, IRB-ready protocols can cut startup timelines by up to 50%, as demonstrated in the ASMD pediatric observational study and the CDKL5 “Patient Voices” initiative.
Key Advantages:
- Digital-first design: Custom eCRFs, eCOAs, eConsent, ePRO, and wearables integration
- Regulatory-grade compliance: CDISC, 21 CFR Part 11, GDPR, HIPAA
- Comprehensive data capture: Clinical, PRO, lab, biomarker, genomic, imaging, payer data
- Lifecycle utility: From natural history, external comparator arms, trial design, long-term follow up to market access and post-marketing
- Efficiency: Up to 40–50% cost reduction with a lean team support model, enabling a faster, more efficient launch than traditional approaches.
Data Without Silos
healthie™ supports high-quality, multi-modal data capture, combining clinical outcomes, patient-reported outcomes, laboratory results, biomarkers, genomic sequencing, imaging (with AI-enabled Metadata integration coming soon), and payer datasets. The critical factor here is how we standardize the data ensuring better data fidelity. By harmonizing these data streams within a single platform, it produces analysis-ready, regulatory-grade datasets without the redundancy and inefficiency of multiple vendor handoffs.
Designed for Reuse
The platform is designed for reusability across the product lifecycle, with transparent pricing and a right-sized support model that scales as needs evolve. From natural history studies, informing trial design and building external comparators to long-term follow-up, and market access evidence generation, healthie™ eliminates one-off study waste. Real-time dashboards, QA/QC automations, and data governance SOPs ensure data integrity and operational transparency throughout.
6. Case Snapshot: Dynamic NHS in Rare Liver Disease
Pulse launched a fit-for-purpose NHS for a sponsor using healthie™’s ready-to-use infrastructure. Leveraging our existing platform and workflows, the project moved from feasibility assessment to a finalized NHS protocol in a significantly reduced timeframe compared to traditional builds. Features included:
- ePROs tailored to the indication and integrated with site workflows
- Multi-site, multi-country IRB management
- Real-time dashboards for recruitment tracking and symptom analysis
Outcome: The registry enabled confident selection of a primary endpoint for a pivotal study, supported a successful FDA Type B meeting, and is now being leveraged for a long-term follow-up program.
Conclusion: Real-World Data with Real-World Impact
A static, siloed, retrospective approach no longer meets the bar for regulatory-grade insights. Biotech’s developing first-in-class or rare disease therapies need agile, extensible, and high-integrity real-world data.
With healthie™, sponsors gain control, speed, and quality, without the cost and complexities associated with the traditional approach. This platform-driven model allows NHS to scale with the program, from preclinical planning through commercialization.
If you are planning a pivotal study or need regulatory-grade data in an under-researched indication, let’s discuss how to design your NHS from day one.
Jim Barrio | Sr. Director, Business Development
jim.barrio@pulseinfoframe.com
Ryan Sheedy | Associate Director, Business Development



