Drug Discovery Central
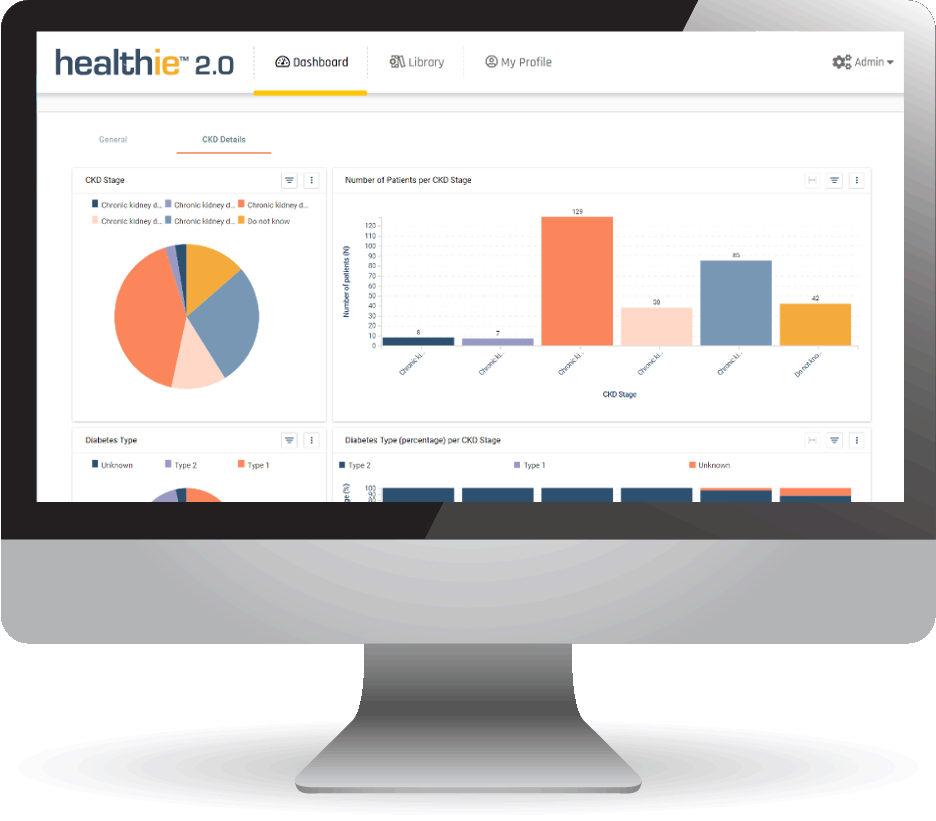
Our globally deployed evidence generation platform enables us to rapidly scale and integrate multiple datasets and data sources, even as regulations evolve. The collective evidence that can be generated from our healthie™ platform can be leveraged for the purpose of, but not limited to:
- Supporting decentralized clinical trials and studies
- Understanding the burden of disease
- Developing treatments and guidelines
- Developing of biomarkers and endpoints
- Leveraging natural history as comparator arm to facilitate new drug development/clinical trials
- Supporting hypothesis generation and publications
- Supporting health economics and outcomes research