HOW TO UPDATE THE NEWS BLOCKS:
- Change background of column to news background (click on pencil with grey background)
- Update title (set to Heading 2)
- Update Learn More button
- If the image is dark enough, remove raw HTML block at bottom
We do not outsource our science. We have an in-house team at Pulse Infoframe whose research and epidemiological expertise is the foundation of everything we develop. They help address the research questions that are top of mind for patients.
We develop trusted relationships with leading researchers, key opinion leaders, advocacy groups, and data partners to deepen our expertise and advance data collection from an ever-expanding number of sites, stakeholders, and data sources.
Our platform adjusts to your needs, wherever in the world members of your community reside and however your needs change. Our time-tested, compliant consenting and governance procedures allow researchers worldwide to access the data your community chooses to share.
Pulse Infoframe is a global healthcare company that has developed a best-in-class real-world evidence platform to support research in oncology, rare disease and other complex conditions, for more than a decade.
Collaborating with leading biopharmaceutical companies, research institutions, and patient advocacy groups, Pulse enables the collection and sharing of high-quality data to support research, regulatory submissions, and advancements in personalized medicine. Pulse’s cloud-based platform enables the integration and analysis of diverse data sets, including the harmonization of novel data collected directly from sites and patients into the platform with existing data from sites, registries and clinical trials.
The Pulse platform empowers stakeholders to generate insights that accelerate drug development, improve patient outcomes, and enhance the understanding of rare and complex diseases, helping to drive innovation in healthcare globally. This work is instrumental in bridging the gap between data and decision-making in healthcare.
The team at Pulse Infoframe brings decades of research, epidemiology, and operational experience from organizations like ConcertAI, Oracle (Cancer Research Cloud), PatientsLikeMe, CureForward, Salesforce, N-of-One, Apple, Optum, and Brigham & Women’s Hospital. As a team, we have 200+ peer reviewed publications.
Our technology platform enables the structured collection of data that is mapped to Observational Medical Outcomes Partnership (OMOP), mCode, and others. We make sure the data captured allows for consented data-sharing permissioning across stakeholders and meets regulatory standards required by the FDA and EMA.
Our evidence generation platform engages patients, caregivers, study coordinators, advocates, and industry. It includes consent management, library with educational materials, repository of patient reported outcome measures, and natural and disease-specific data elements that can be easily deployed.
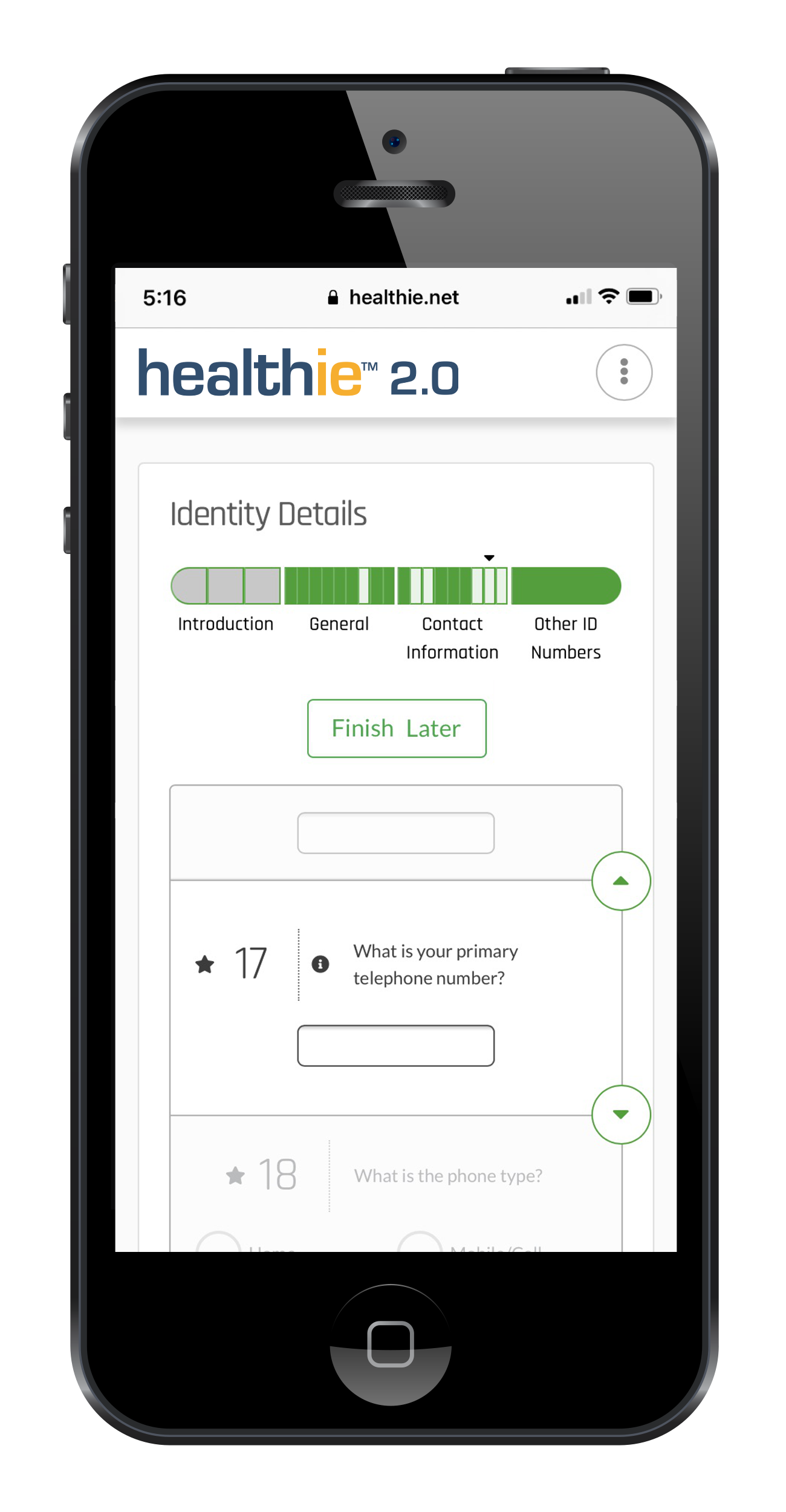
Our technology platform enables the structured collection of data that is mapped to Observational Medical Outcomes Partnership (OMOP), mCode, and others. We ensure the data captured allows for consented data-sharing permissioning across stakeholders and meets regulatory standards required by the FDA and EMA.
Since Pulse Infoframe’s inception, we have leveraged our deep domain expertise to develop the industry’s most robust evidence generation platform and support longitudinal prospective studies and registries in over 25 diseases such as melanoma, lung cancer, and CDKL5 in partnership with over 10 global pharma partners seeking to understand natural history of disease, treatment effectiveness, and burden of illness. In short: We know our stuff.
These are the domains we specialize in and for which we’ve developed a platform to help your community around the world collect and share its data and lived experiences. More voices equals stronger advocacy and a greater possibility for the development of effective treatments.





